advance the field of rheumatology

Neuroimmune Modulation changes the equation in RA
The introduction of biologics over two decades ago was a major development in the management of chronic autoimmune conditions like rheumatoid arthritis. The SetPoint System expands your treatment armamentarium by offering you and your patients the first and only neuroimmune modulation therapy.
Revolutionize patient care — and your practice
The convergence of extraordinary advances in immunology, neuroscience, and engineering have resulted in the development of an entirely new treatment approach for rheumatoid arthritis, the SetPoint Therapy.
Breakthrough treatment option with novel mechanism of action
The SetPoint System activates the body’s innate neuroimmune pathways to restore immunologic homeostasis by modulating intracellular pathways to reduce production of an array of proinflammatory cytokines1 , 4—providing a novel treatment option for your biologic-experienced RA patients.
Safe and proven treatment for biologic-experienced RA
SetPoint’s unique mechanism of action builds on over two decades of engineering and clinical experience with devices to stimulate the vagus nerve—providing you with a safe and efficacious treatment option to offer patients with moderately to severely active RA who are inadequate responders or intolerant to biologics or JAK inhibitors.
Control of therapy choices is back in your hands
As the only approved medical device for RA, you can offer your patients SetPoint Therapy while preserving all your therapeutic options. The unique mechanisms of action of SetPoint Therapy means that you can combine it with a biologic or a JAK inhibitor, if needed.

Modulating the immune system with precise neurostimulation
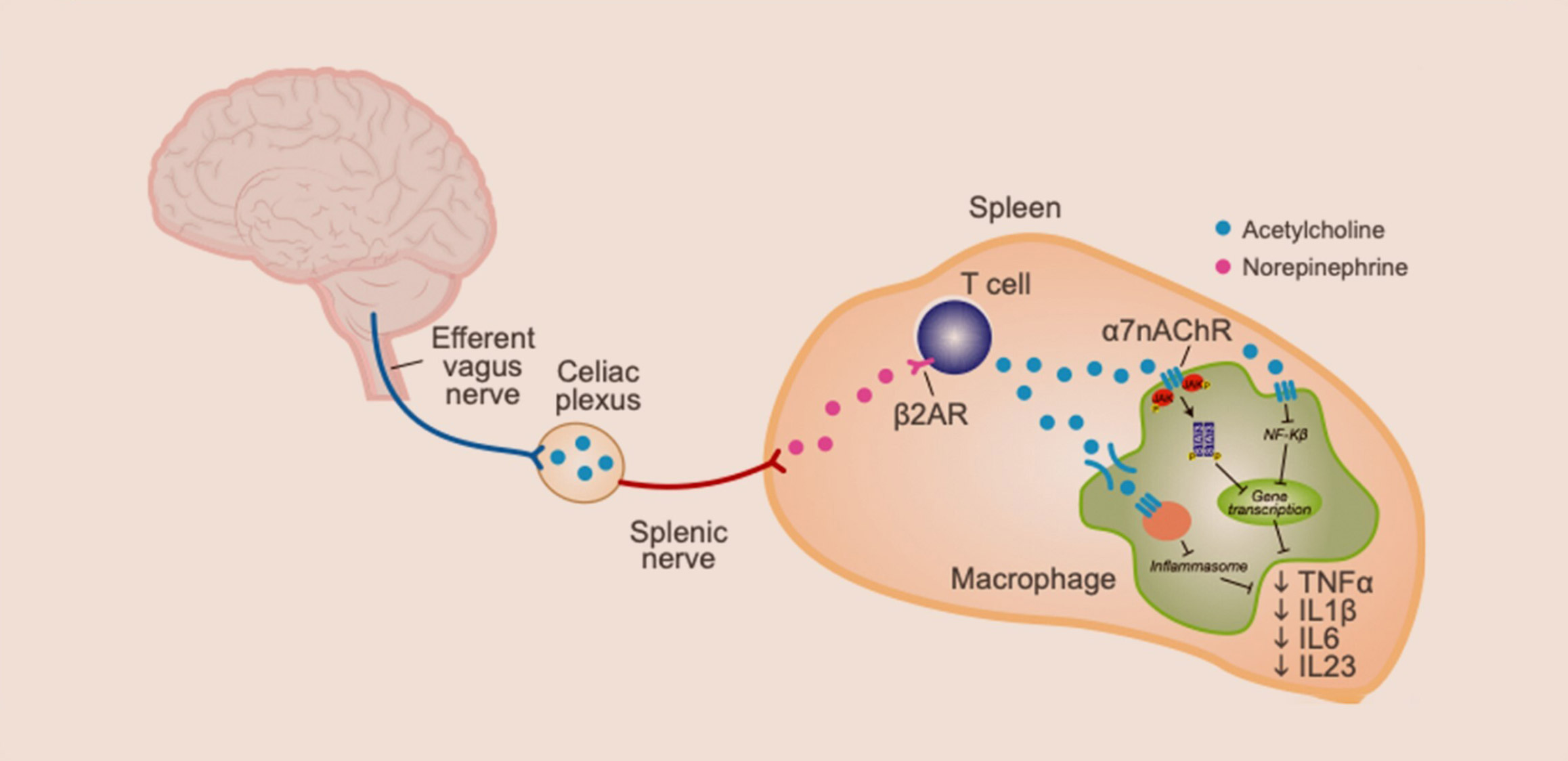
The central nervous system governs homeostatic control of both immune responses and bone turnover through innate, vagus nerve-mediated neuroimmune and osteo-targeted reflexes.1,2
Electrically stimulating specific fibers of the vagus nerve5,6 can activate the anti-inflammatory and immune-restorative pathways that result in acetylcholine release and specific agonism of the α7 nicotinic acetylcholine receptors on immunocytes.3,4,5
Subsequent modulation of intracellular processes acting through NFkB, JAK/STAT, and the inflammasome pathways4,5 leads to 30-70% reduction in the production of an array of proinflammatory cytokines like TNF, IL-6, IL-1β.6,7 This broad-spectrum immunomodulation retains cytokine network bioavailability, thereby allowing reduction of inflammation while maintaining competent immunosurveillance against infections and malignancies,4,5 and without increasing the risk of major cardiac events.
Activating the evidence
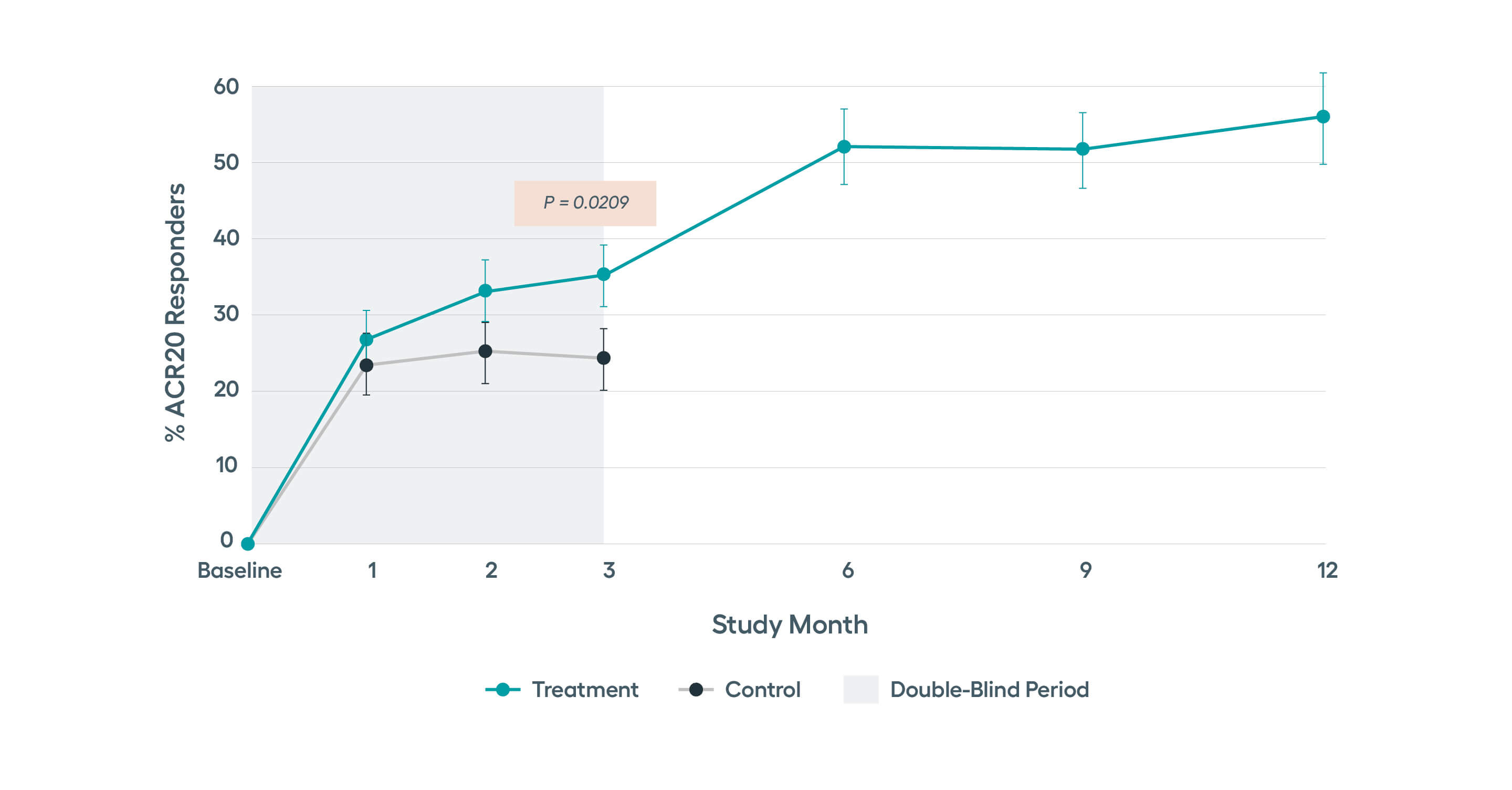
The only medical device proven to be effective and safe for the treatment of RAModified non-responder imputation (NRI) data. Patients were imputed as non-responder if rescued prior to Month 3, regardless of treatment assignment. After Month 3, data presented for patients on stimulation monotherapy, i.e. excludes data from patients if stimulation treatment was combined with high-dose steroid or b/tsDMARD (n in treatment arm, 3 months= 122, 6 months = 96, 9 months = 89, 12 months =77).
How to add SetPoint Therapy to your practice
SetPoint Medical is committed to making it easy for you to add neuroimmune modulation to your RA treatment armamentarium. Get started by contacting your SetPoint Territory Manager. They will walk you through the steps below to ensure that you and your practice are ready to offer your biologic-experienced RA patients this revolutionary treatment option.
Here are the steps to Start Activating
STEP 1: Surgical Partnership
Your SetPoint Territory Manager will set up an introductory meeting with a surgeon who has been trained on the SetPoint Implant.
STEP 2: Practice Integration
Your Territory Manager will provide you and your team with the training and materials you’ll need to identify appropriate RA patients for SetPoint Therapy.
STEP 3: Patient Education
SetPoint Medical will supply comprehensive resources to help you educate your patients on neuroimmune modulation. The SetPoint Hub will provide you with a complete pathway for efficient care, coordination, and patient engagement throughout the referral, prior authorization, and Implant placement process.
STEP 4: Prior Authorization Support
SetPoint’s commitment to seamless adoption of neuroimmune modulation means that we will work directly with the surgical facility to seek prior authorization for the SetPoint Therapy on your patient’s behalf. Our navigation team will keep you and your patients informed to ensure a best-in-class experience for your patients and your staff.
STEP 5: Activate Therapy
Patients return to your office a few days after their Implant placement procedure to activate their therapy. Your Territory Manager will be on-hand to walk your staff through the activation procedure using an iPad-based application.
STEP 6: Center of Excellence
As one of the first providers of this novel therapy in the country, you will be on the forefront of activating this new therapy in your practice.

Connect With Us

Resources



Frequently asked questions about the SetPoint System
How does the SetPoint System work?
The SetPoint System is a neuroimmune modulatory device that treats RA and is designed to restore immunologic homeostasis. 3,4,8 The SetPoint System consists of an integrated neurostimulator intended to activate innate anti-inflammatory processes to modulate multiple intracellular pathways,3,4,5 thereby reducing production of an array of proinflammatory cytokines6,7—providing a novel treatment option indicated for your biologic-experienced RA patients. The stimulation parameters of the SetPoint System are based on extensive preclinical biomarker analysis that indicated that a single 60-second daily stimulation reduces multiple cytokines, including TNF-α, IL-β, and IL-6, by 30-70%, providing symptom reduction without immunosuppressive risks.4,5,6,7
Is the SetPoint System safe and effective for treatment of RA?
The SetPoint System’s unique mechanism of action builds on over two decades of research and engineering, including the clinical experience from other devices that stimulate the vagus nerve, to provide you and your patients with the first neuroimmune modulation therapy for treatment of RA.
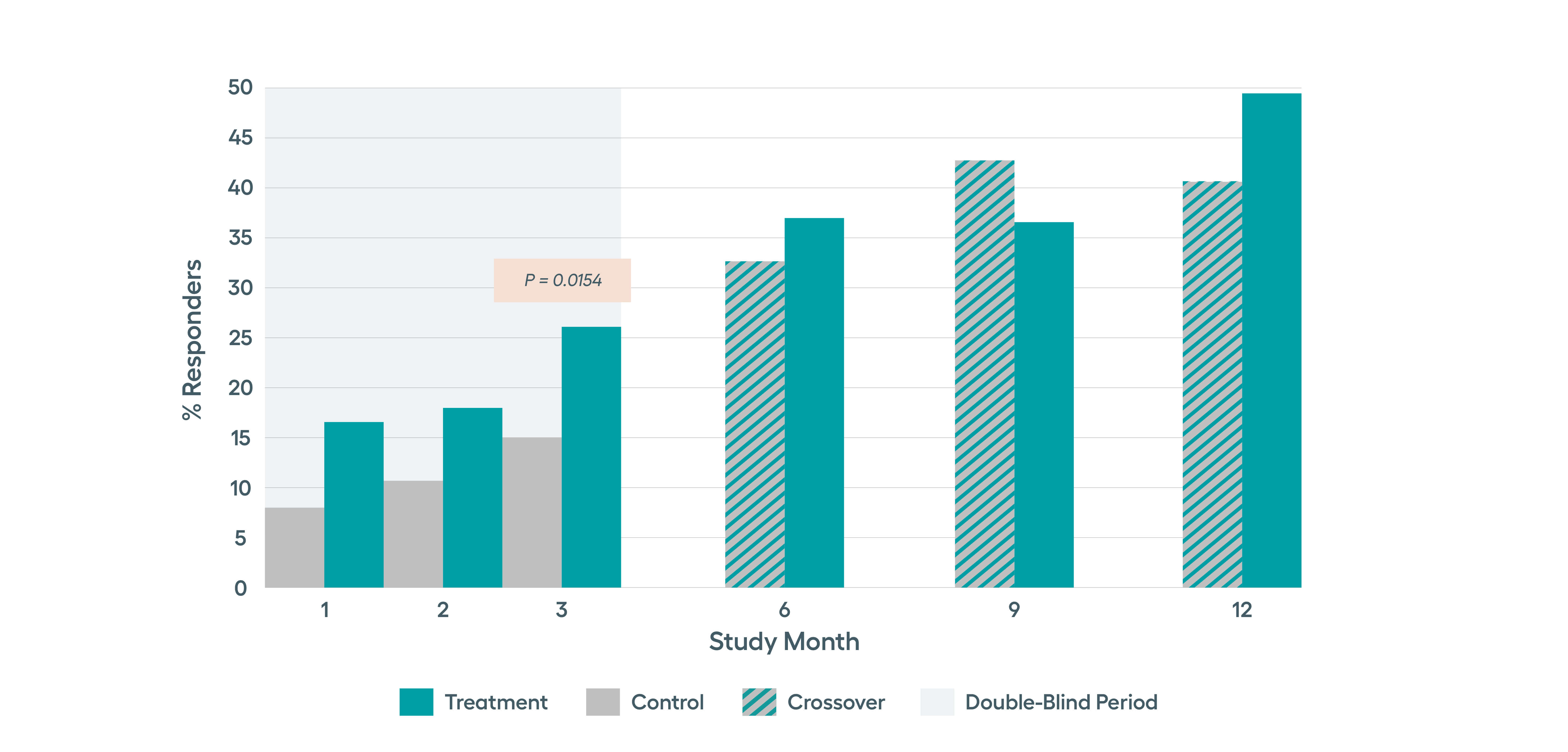
The SetPoint System’s safety and effectiveness were studied in the RESET-RA clinical study that enrolled 242 patients who were inadequate responders or intolerant to one or more biologics and/or JAK inhibitors. The study successfully met its primary endpoint of ACR20 at 3 months, with sustained improvements observed through 12 months of follow-up compared to baseline. With 12 months of therapy, nearly half of the patients were in DAS28-CRP low disease activity or remission.*
The SetPoint System, as well as the implant procedure, is considered safe.10 There was a low risk (1.7% n = 4) of related serious adverse event rate in the peri-operative 3 months, with no (0%) related serious events occurring through 12 months. Additionally, through 12 months, there were no malignancies, major cardiac events, or serious infections associated with the SetPoint System, highlighting the safety of this novel therapeutic option.
To see detailed data, visit the Clinical Evidence page.
*Analysis based on patients who were treated with SetPoint Therapy for 12 months without the addition of biologic, JAK inhibitors or high-dose steroids, or change in csDMARDs.
Who is the ideal patient candidate for SetPoint Therapy?
The SetPoint System is indicated for adults with moderately to severely active RA who have had an inadequate response, loss of response, or intolerance to one or more biological or targeted synthetic DMARDs.
Hence, the SetPoint Therapy could be considered for adult RA patients who have failed at least one biologic or JAK inhibitor and are seeking an alternative therapy; those who cannot tolerate these medications; those who are at high safety risks for JAK inhibition due to comorbid conditions; and those who had failed to get adequate disease control on biologics or JAK inhibitors.
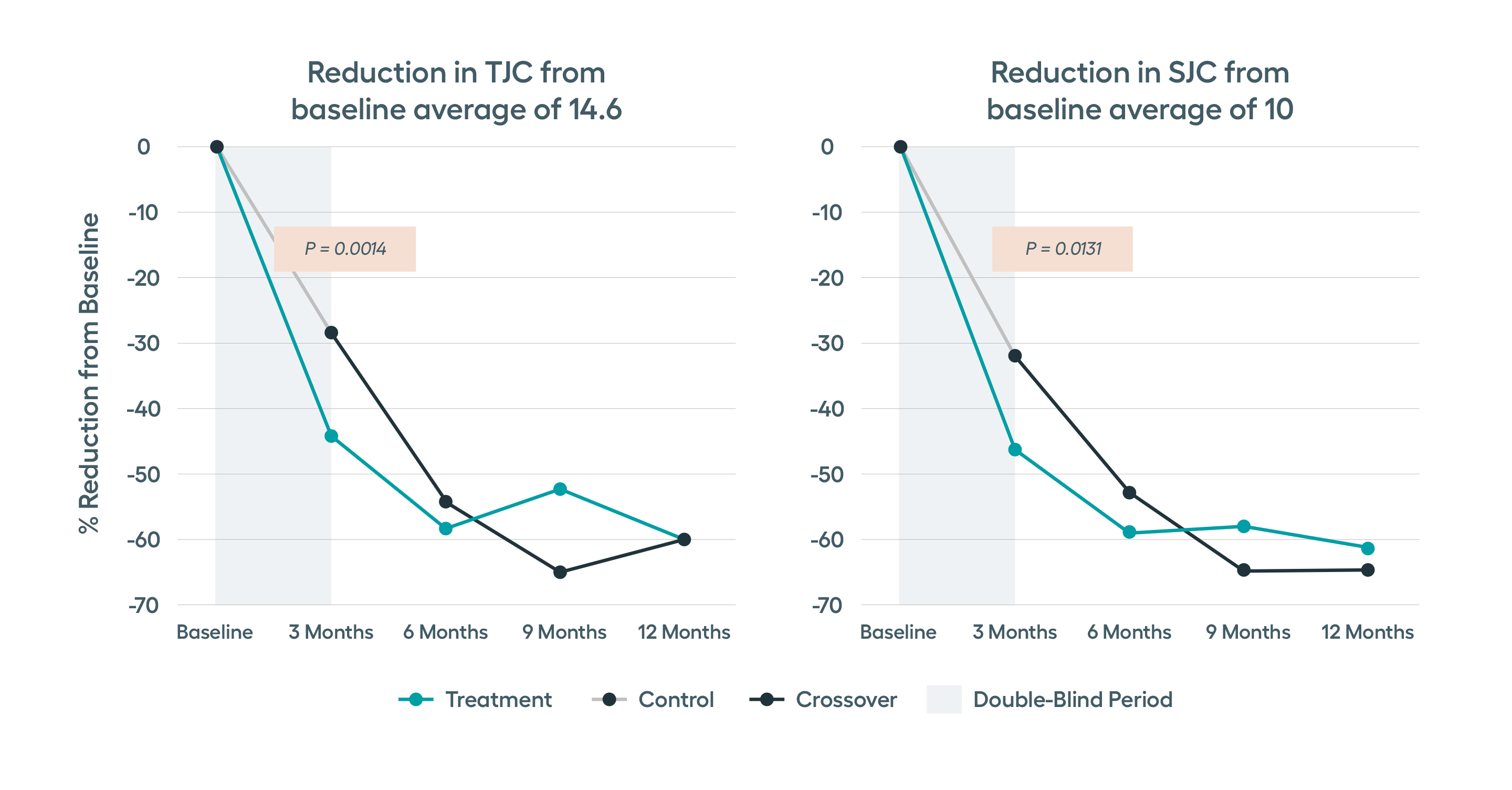
In the RESET-RA study, improvement in the number of tender and swollen joints as well as disease activity was seen by 3 months after starting stimulation. This response rate was higher in patients who had exposure to just one prior biologic.
Based on its mechanism of action that manages the inflammatory process without immune suppression, SetPoint Therapy can be combined with biologics or JAK inhibitors if desired, preserving treatment options in the future.3,4,5,8
Do patients feel the implant or stimulation?
SetPoint Therapy is programmed to ensure comfort for the patients. Activation of the device includes a gradual increase of the stimulation level to ensure the therapy is not uncomfortable. Sensation of stimulation varies, but patients may feel a light tickling sensation, cough, or changes in voice during stimulation. The stimulation is delivered automatically for 60 seconds each day, and the rheumatologists can personalize the time of stimulation.
The SetPoint Implant typically cannot be felt or palpated externally.
What infrastructure does my clinic need to offer SetPoint Therapy to my RA patients?
We are committed to making it easy for you to offer SetPoint Therapy to your RA patients. Our Territory Manager will support your clinic’s onboarding process and can connect you with SetPoint-trained surgeons in your area.
Upon your clinic’s activation, SetPoint Medical will provide you and your team with the training and materials you’ll need to educate your RA patients on this therapy. SetPoint Medical will work directly with the surgical facility to help seek prior authorization for the procedure on your patient’s behalf. SetPoint Activators are available to help your patients navigate through the referral, prior authorization, and implant placement processes.
A couple of weeks after the implant procedure, the patient will return to your office to have therapy activated using an iPad-based app, which SetPoint Medical will provide. After activation, the therapy is delivered automatically for 60 seconds each day.
If you have additional questions, please contact your SetPoint Territory Manager.
What is the implant procedure like?
The SetPoint System is typically placed during a 60-to-90 minute outpatient surgical procedure under general anesthesia.9 Patients typically go home the same day as the procedure and resume activities of daily living the following day.
The SetPoint Implant is placed by a surgeon who is experienced with procedures involving the vagus nerve, typically a functional neurosurgeon or an ENT surgeon. Your Territory Manager can help to identify trained surgeons near you. Additionally, SetPoint Medical will assist with the prior authorization process for the SetPoint procedure and will keep you and your patients informed to ensure a best-in-class experience for your patients and your staff.
If you have additional questions, please contact your Territory Manager.
How does the SetPoint System work?
The SetPoint System is a neuroimmune modulatory device that treats RA and is designed to restore immunologic homeostasis. 2,3,7 The SetPoint System consists of an integrated neurostimulator intended to activate innate anti-inflammatory pathways to modulate multiple intracellular pathways,2,3,4 thereby reducing production of an array of proinflammatory cytokines5,6—providing a novel treatment option indicated for your biologic-experienced RA patients. The stimulation parameters of the SetPoint System are based on extensive preclinical biomarker analysis that indicated that a single 60-second daily stimulation reduces multiple cytokines, including TNF-, IL-1, and IL-6, by 30-70%, providing symptom reduction without immunosuppressive risks.3,5,6
Who is the ideal patient candidate for SetPoint Therapy?
The SetPoint System is indicated for adults with active, moderate-to-severe RA who have had an inadequate response, loss of response, or intolerance to one or more biological or targeted synthetic DMARD.
Hence, SetPoint Therapy is ideally suited for RA patients who do not want to continue or cannot tolerate biologics and/or JAK inhibitors, those who are at high safety risks for JAKs due to comorbid conditions, and those who had failed to get adequate disease control on biologics and JAKs.
Pre-specified analysis of the safety and efficacy data from the RESET-RA study in biologic-experienced RA patients indicated that therapy response rates are faster and higher among patients with fewer prior biologic exposure, with those with just one prior biologic exposure having most robust responses.
Uniquely, due to its mechanism and safety profile, SetPoint Therapy can be combined with biologics or JAK inhibitors, if desired, preserving all of your treatment options in the future.
Do patients feel the implant or stimulation?
The SetPoint Therapy is programmed to ensure comfort for the patients. Activation of the device includes gradual increase of the stimulation current to ensure that the therapy is never uncomfortable. Sensation of stimulation varies, but patients may feel a light tickling sensation, cough, or changes in voice during stimulation – that is delivered automatically for just 60 seconds each day.
The SetPoint Implant cannot be felt or palpated externally.
What is the implant procedure like?
The SetPoint System is typically placed during a 60-90 minute outpatient procedure under general anesthesia. Patients typically go home the same day as the procedure and resume activities of daily living the following day.
The Setpoint Implant is placed by a surgeon who is experienced with procedures involving the vagus nerve, typically a functional neurosurgeon. Your SetPoint Territory Manager will identify and train a neurosurgeon near you to ensure a seamless referral experience for you and your patient. Additionally, SetPoint will obtain prior authorization for the SetPoint procedure and will keep you and your patients informed to ensure a best-in-class experience for your patients and your staff.
If you have additional questions, please contact your SetPoint Territory Manager.
How do you place the SetPoint Implant?
The SetPoint System includes a small, leadless, integrated neurostimulator that is placed directly on the left vagus nerve through a single incision during an outpatient procedure. The leadless implant is placed on the nerve within a flexible silicon Pod.
Once the vagus nerve is accessed, the Pod is first placed under the nerve. The Implant is then placed in the Pod, which is then sutured closed. An integrated, leadless implant with rechargeable battery means there is no need to coil electrodes around the nerve, to tunnel the lead or create a chest pocket for a separate battery or generator.
Please see complete placement instructions in the Surgical Manual
2. Bajayo A, et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci U S A. 2012;109(38):15455-15460.
3. van Maanen MA, et al. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(4):229-232.
4. Kelly MJ, et al. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep Med. 2022;3(7):100696.
5. Chavan SS, et al. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity. 2017;46(6):927-942.
6. Levine YA, et al. Harnessing the Inflammatory Reflex for the Treatment of Inflammation-Mediated Diseases. Cold Spring Harb Perspect Med. 2020;10(1):a034330.
7. Genovese MC, et al. Safety and efficacy of neurostimulation with a miniaturised vagus nerve stimulation device in patients with multidrug-refractory rheumatoid arthritis: a two-stage multicentre, randomised pilot study. Lancet Rheumatol. 2020;2(9):e527-e538.
8. Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853-859.
9. Peterson D, et al. Clinical safety and feasibility of a novel implantable neuroimmune modulation device for the treatment of rheumatoid arthritis: initial results from the randomized, double-blind, sham-controlled RESET-RA study. Bioelectronic medicine. 2024 10:8.10. Data on file at SetPoint Medical.