Neuroimmune modulation changes the equation in ra

The only medical device proven to be safe and effective for the treatment of RA
SetPoint Medical is committed to evidence-based advancement of its platform for the treatment of RA. The SetPoint System is a novel device-based treatment option for patients who have tried and failed at least one biologic or targeted-synthetic DMARD.
Proven to be safe
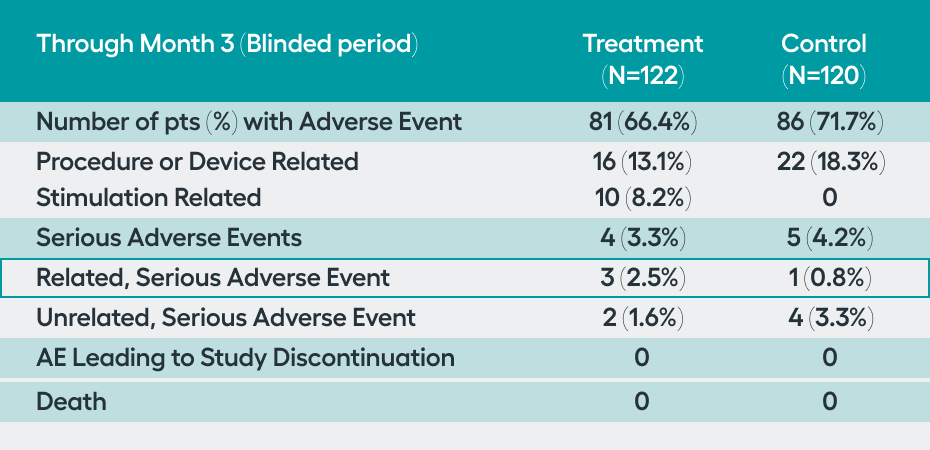
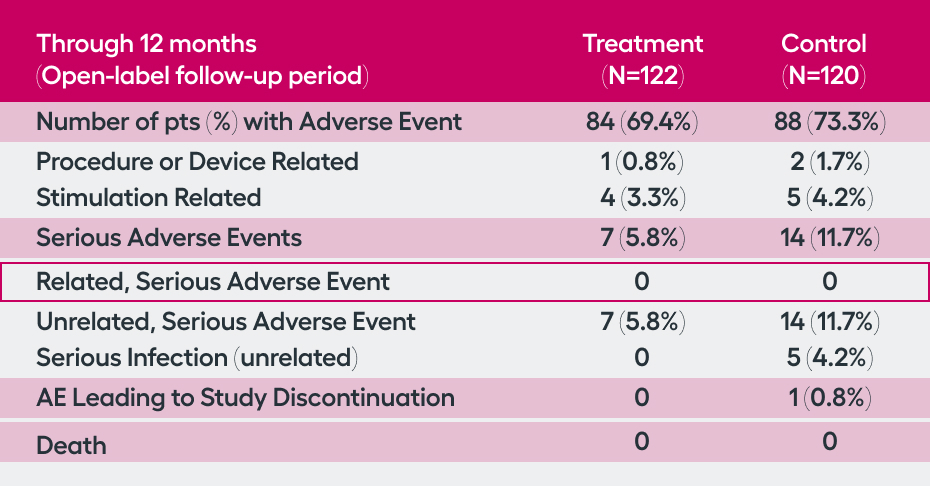
SetPoint System, and its placement procedure, were considered safe. There was a low rate (1.7%, n=4) of related serious adverse events in the peri-operative 3 months, with no (0%) related serious adverse events occurring through 12 months. Additionally, through 12 months, there were no malignancies, cardiac issues, or serious infections considered to be related to the SetPoint System, highlighting the safety of this novel therapeutic option.
Effective in improving disease activity
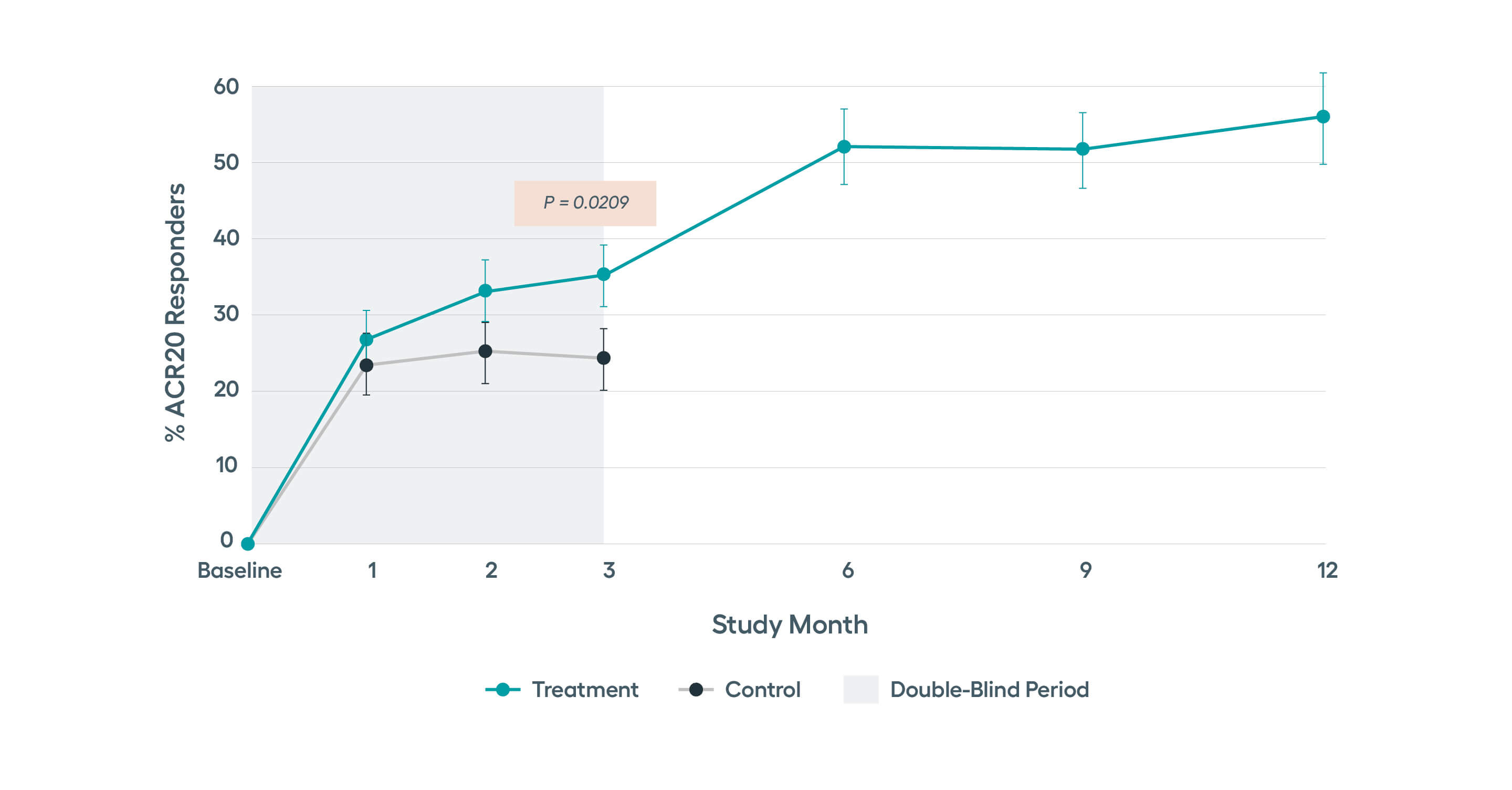
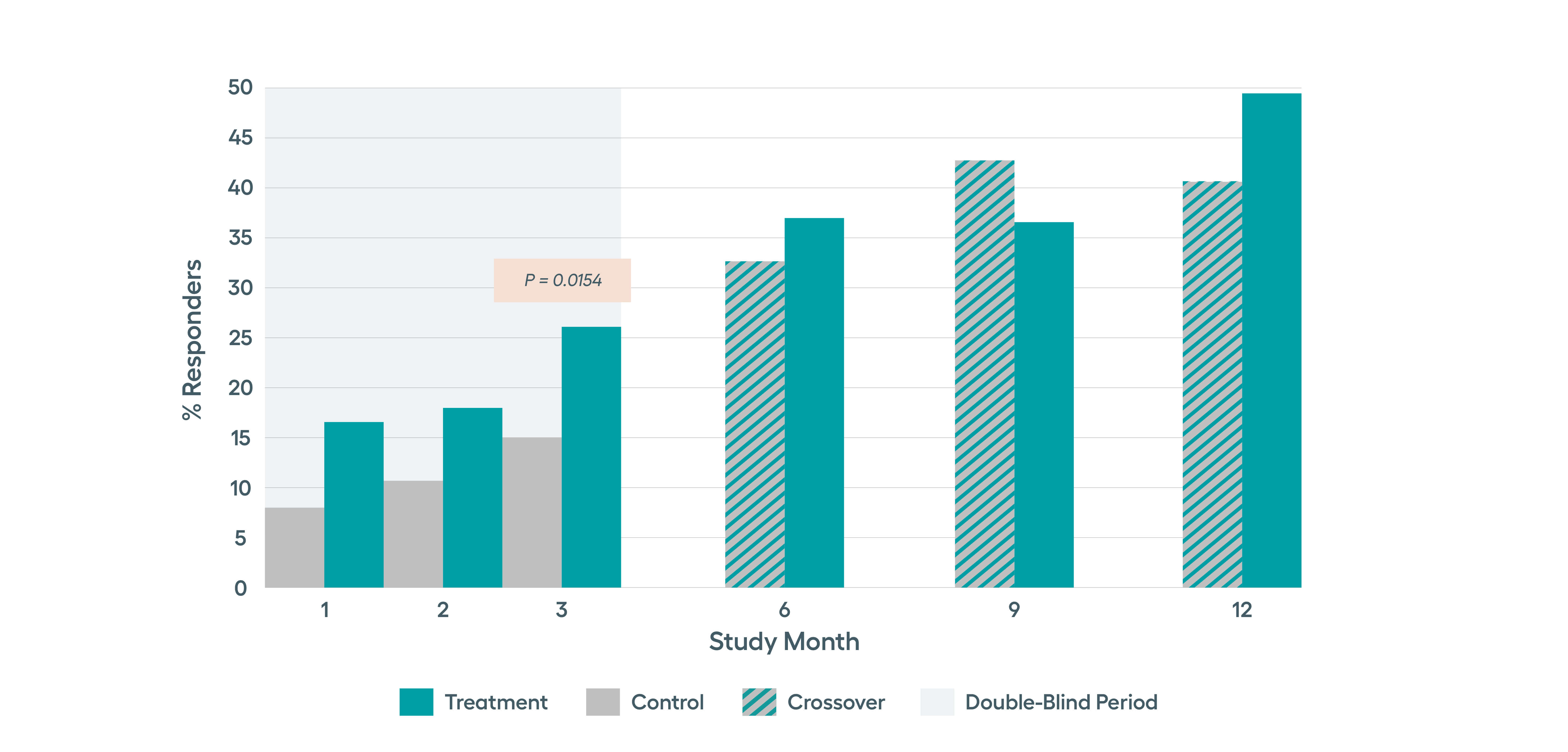
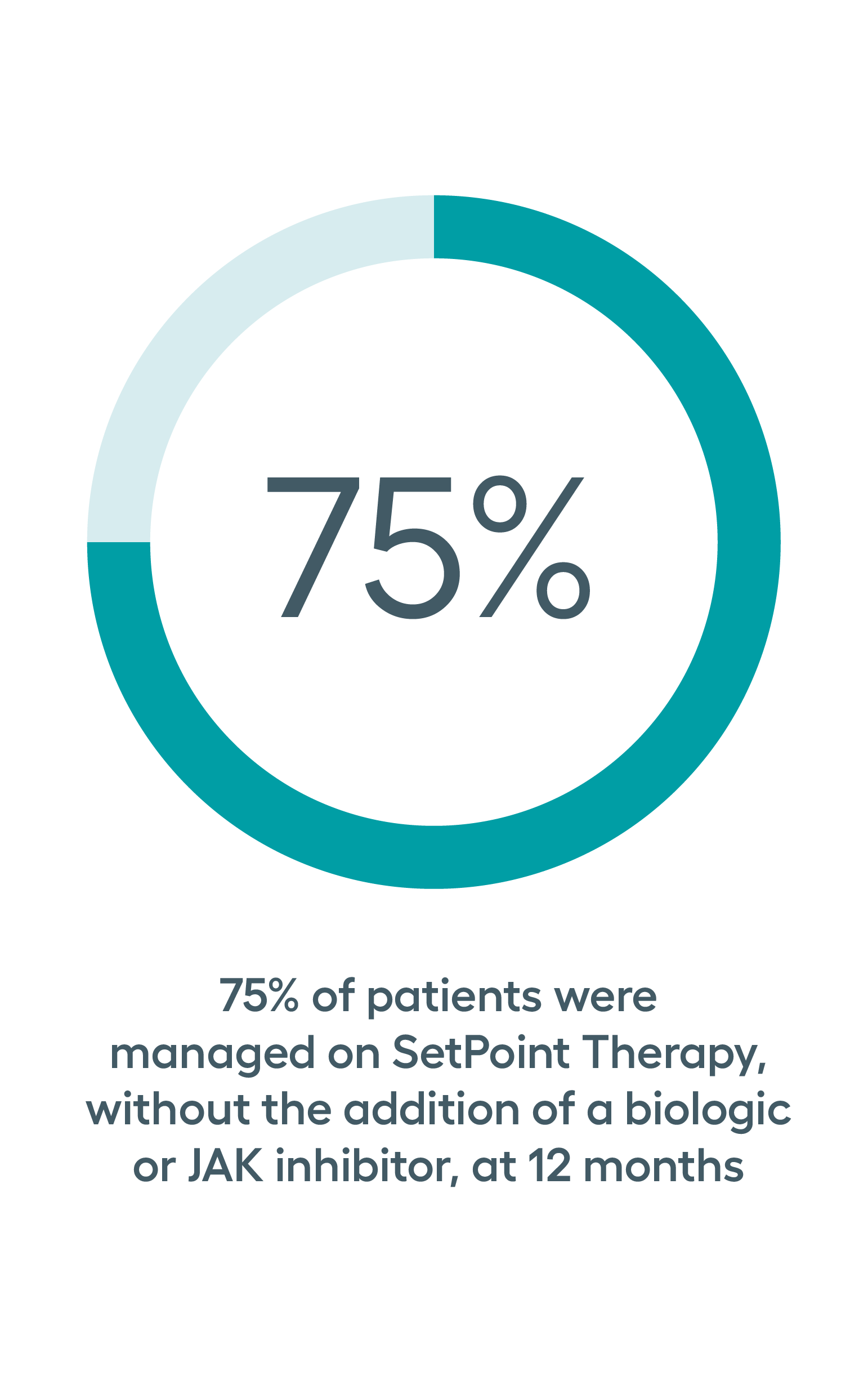
SetPoint System’s effectiveness was studied in the RESET-RA study which met its primary effectiveness endpoint of ACR20 at three months, with sustained improvement observed in ACR response rates through 12 months of follow-up. Approximately 50% of patients on SetPoint Therapy for 12 months achieved DAS28-CRP low disease activity or remission.*
*Analysis based on patients who were treated for 12 months with SetPoint Therapy without the addition of a biologic, JAK inhibitor or steroids, or change in their csDMARD.
Protection from structural damage
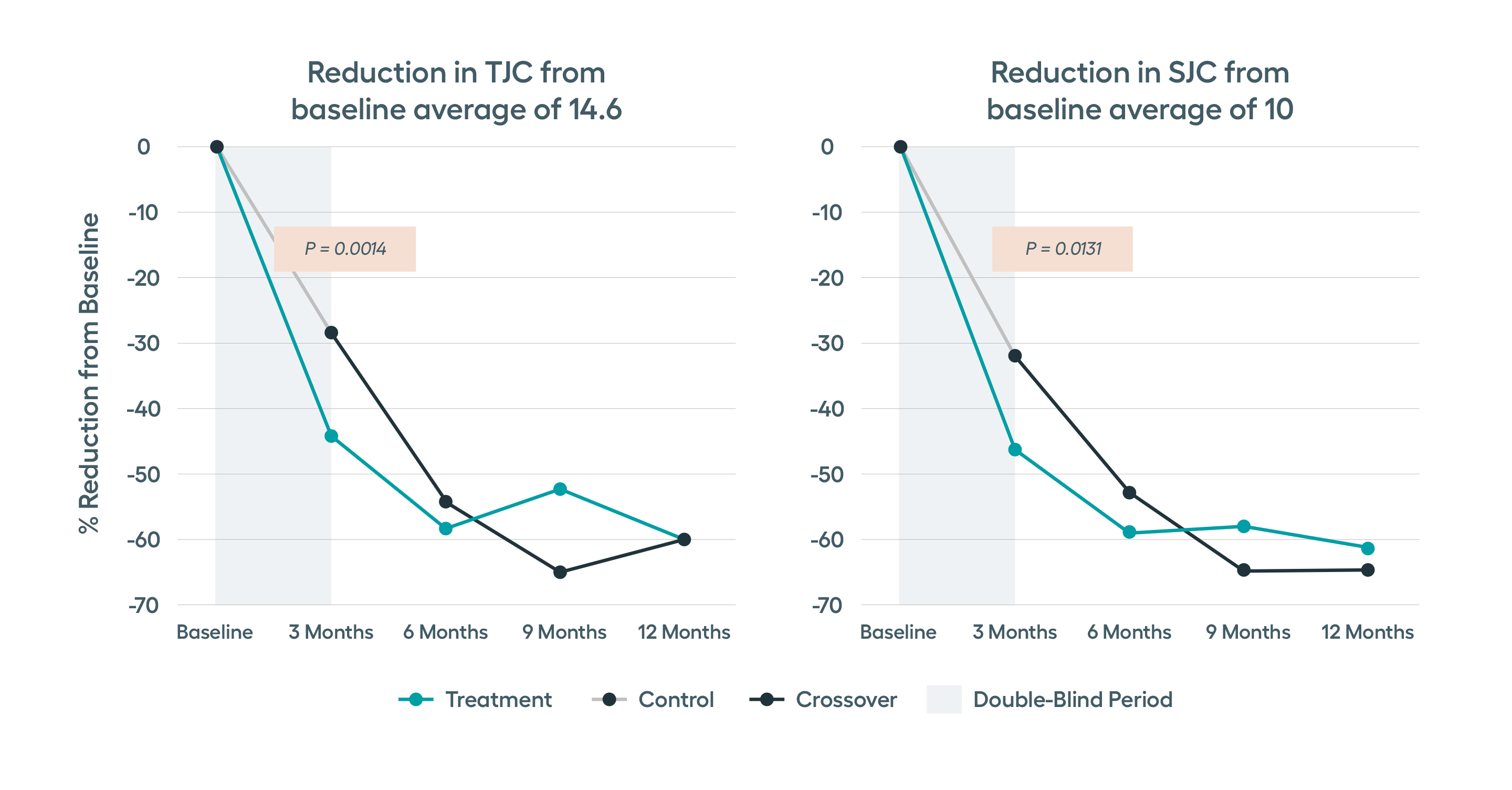
SetPoint Therapy provides protection from joint erosion progression as early as three months following device activation, as observed by MRI. Statistically significant improvement in tender and swollen joint counts seen as early as 3 months, with continued improvement through 12 months.
Improved therapy persistence
98% of participants in the RESET-RA study remained on their SetPoint Therapy through 12 months. 78% reported satisfaction with their therapy, with 94% indicating they would recommend it to their family or friends.

How effective is the SetPoint System for treatment of RA?
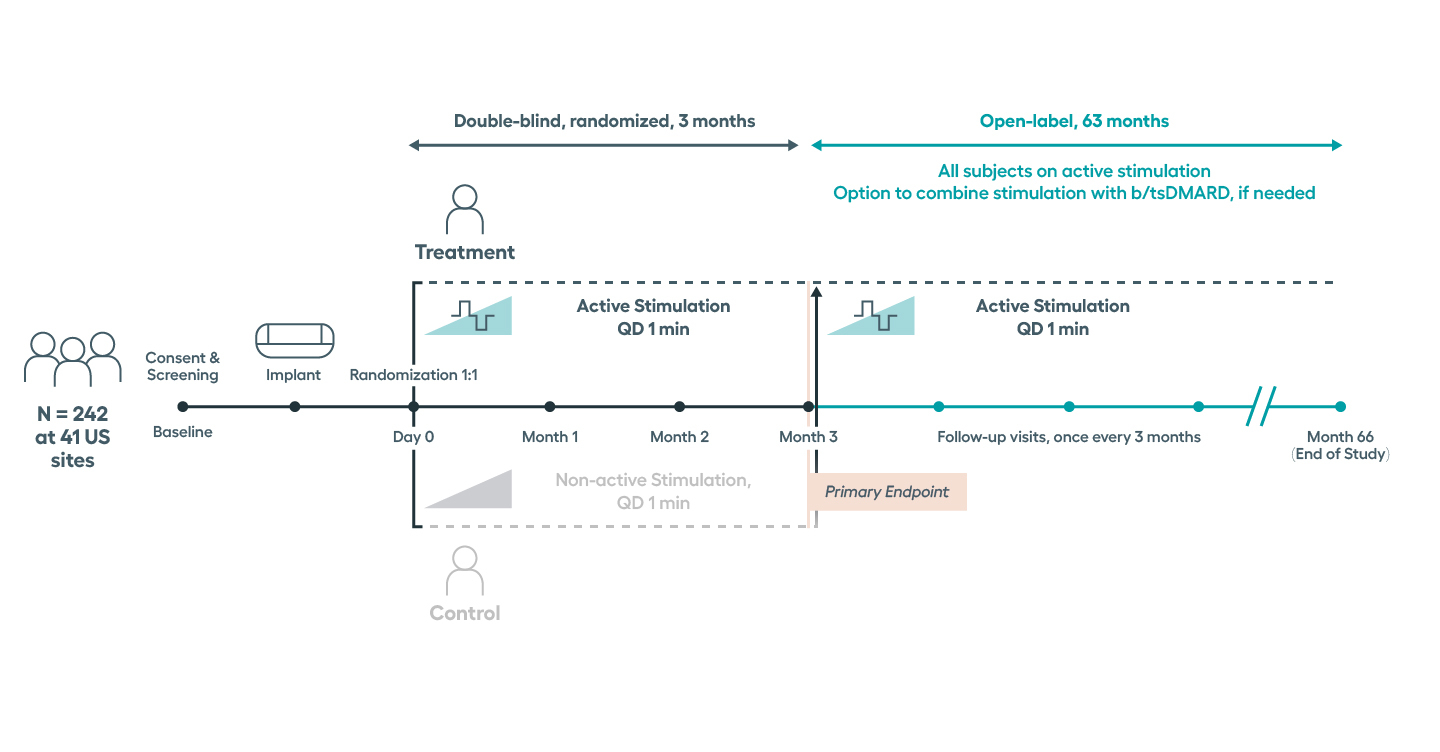
Effectiveness of the SetPoint System was evaluated in the RESET-RA study, a randomized, blinded, controlled, pivotal trial. The RESET-RA study demonstrated that the SetPoint System was a safe and effective treatment option for adults with moderate-to-severe RA who have had an inadequate response, loss of response, or intolerance to one or more prior biologic or targeted synthetic DMARDs—including Janus Kinase (JAK) inhibitors.
Activating the evidence
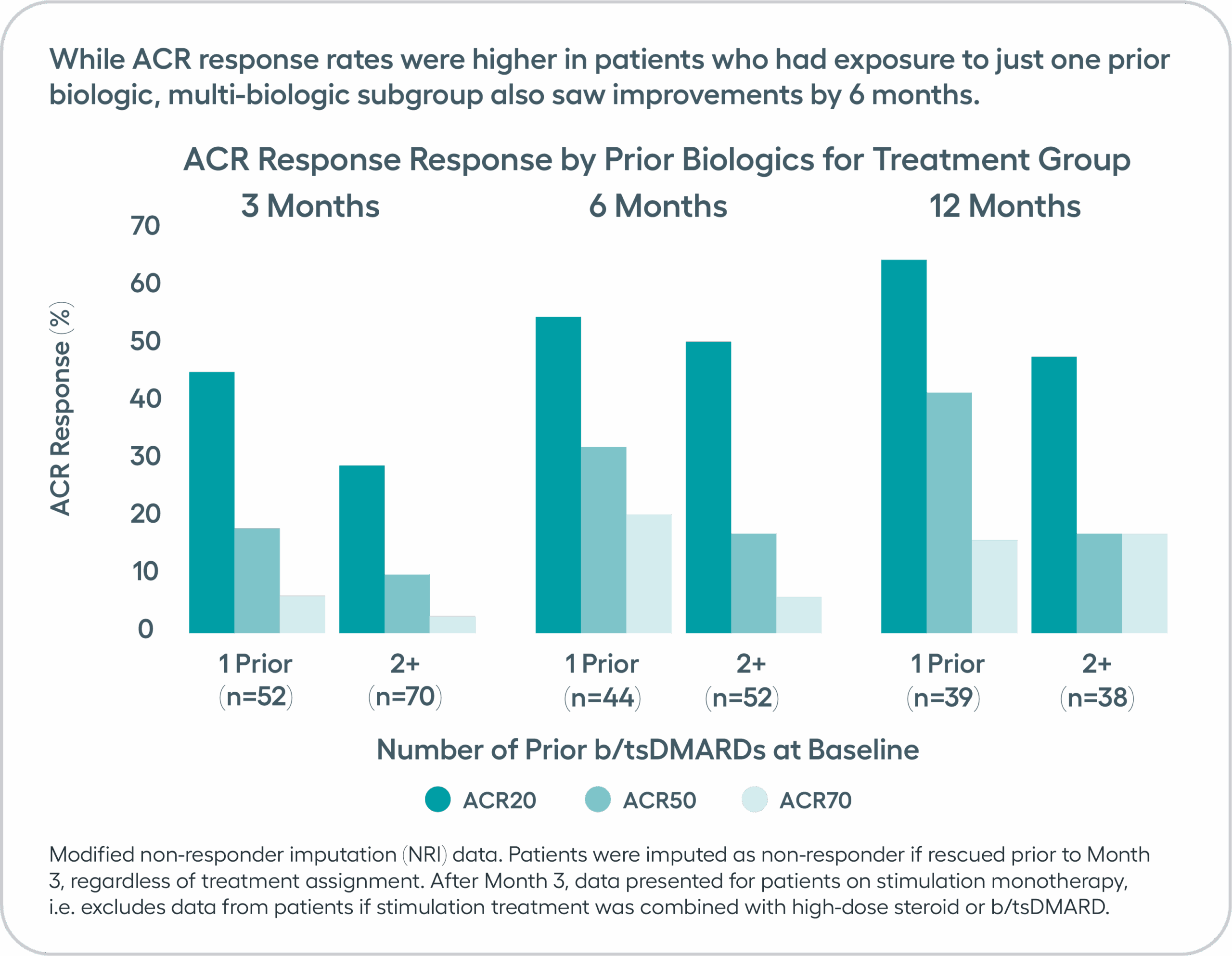
Significant improvements in tender and swollen joint counts, ACR20 and disease activity observed at 3 months, with sustained improvements through 12 months.Modified non-responder imputation (NRI) data. Patients were imputed as non-responder if rescued prior to Month 3, regardless of treatment assignment. After Month 3, data presented for patients on stimulation monotherapy, i.e. excludes data from patients if stimulation treatment was combined with high-dose steroid or b/tsDMARD (n in treatment arm, 3 months= 122, 6 months = 96, 9 months = 89, 12 months =77).
Study highlights
Download the publications highlighting the mechanism of action, safety, and effectiveness of SetPoint’s neuroimmune modulation therapy.
Seminal study results
Protection from structural damage as measured by RAMRIS at three months
The RESET-RA collected gadolinium enhanced hand and wrist MRIs to assess joint structure protection. Significant suppression in the progression of bone erosions was observed in the erosive subgroup of patients who had active synovitis and/or osteitis at baseline. Similar improvements were also observed in the subgroup of patients who had failed only one prior biologic or JAK inhibitor prior to entering the study. The proportion of participants with progression of bone erosions favored the stimulation group, although not statistically significant for ITT population.
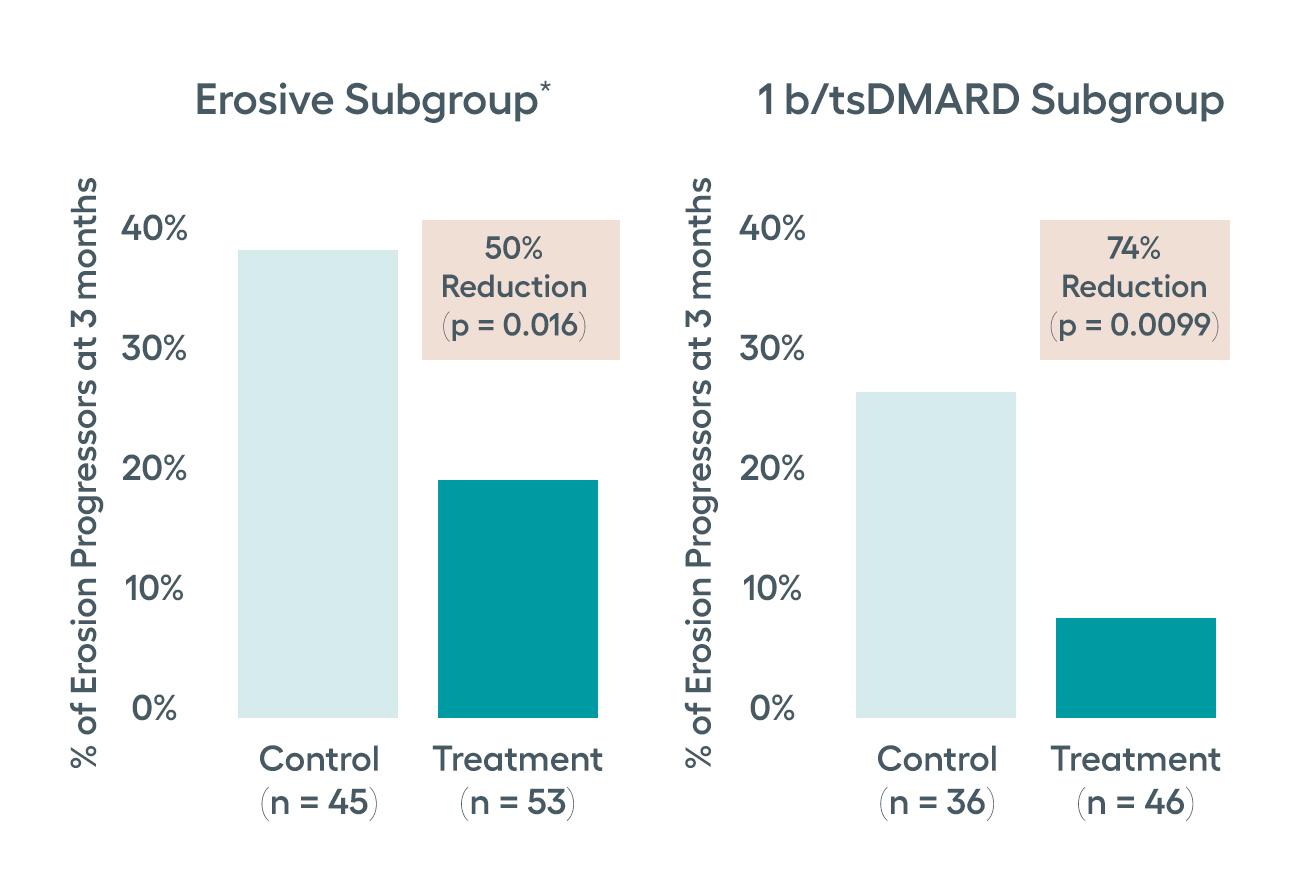
Erosive Subgroup definition: Baseline synovitis score of two or more on any joint; or having at least four joints with a synovitis score of one at baseline; or having any joint with osteitis at baseline.
Patients treated with SetPoint System report strong therapy persistence
Automatic stimulation through an implanted device, coupled with SetPoint’s unique mechanism of action, resulted in 98% therapy persistence through 12 months. Automatic stimulation ensures consistent therapy delivery each day.
A uniquely safe therapeutic option for RA patients
While the SetPoint System is the first device proven safe for treatment of RA, devices to stimulate the vagus nerve have been implanted in 100,000 patients for other neurologic indications over the past 20 years. The RESET-RA study demonstrated that the procedure to place the SetPoint system and the stimulation therapy are well tolerated:
of patients experienced a related, serious adverse event—primarily in the three-month perioperative period.
malignancies, major cardiac events, or serious infections associated with SetPoint Therapy were observed through 12 months of follow-up.

For complete listing of the safety data, please see the User Manuals
Latest publications
Connect With Us