Patients
How does the SetPoint System work?
SetPoint Therapy is a different approach to treating RA when compared to traditional drug-based treatment options.
SetPoint Therapy is a device-based therapy that works with your body by activating your body’s natural immune pathways known to have an anti-inflammatory effect.4 These immune pathways are thought to be dysfunctional in RA patients, which causes uncontrolled inflammation, the root cause of RA.3
The SetPoint System includes placement of a device on your vagus nerve with the intent to jumpstart, or activate, these pathways to reduce inflammation and restore your body’s inflammatory balance, or setpoint. In comparison, the typical approach of biologics and JAK inhibitors is to block/inhibit or target specific inflammatory markers or pathways.
Here is how Lynn, who participated in the RESET-RA study, describes how the therapy works: “This is a natural process. It’s just stimulating a part of your body. It’s telling it to say, hey, we need you to work.”
Hear Lynn’s experience with SetPoint Therapy.
Actual patient stories shared with their permission. Individual outcomes vary. Click here to see the full Important Safety Information.
Is the SetPoint System safe and effective?
Yes, the SetPoint System has been clinically proven to be safe and effective. Specifically, the SetPoint System is indicated for use in the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response, loss of response, or intolerance to one or more biological or targeted synthetic disease-modifying antirheumatic drugs such as TNF inhibitors or JAK inhibitors.
To support its FDA approval, the SetPoint System was evaluated in a clinical study of 242 patients which successfully met its primary endpoint for efficacy, and the device, its placement, and stimulation therapy were considered safe. The study now includes data through 12 months of follow-up that demonstrates sustained improvements in tender and swollen joints as well as disease activity; nearly half of patients treated with SetPoint Therapy for 12 months are in low disease activity or remission.*
The study also demonstrated that SetPoint System, its placement procedure as well as stimulation were safe and well tolerated. Serious adverse events related to the placement of the SetPoint System were observed in 1.7% (4 of the 242 people implanted) in the peri-operative 3 months, and no (0%) additional related serious events through 12 months of long-term follow-up.
SetPoint Therapy is intended to reduce inflammation by activating the body’s natural pathways while preserving the immune system’s primary protective functions.4,5 In the study, there were no serious infections, cancers, or major cardiac events associated with SetPoint Therapy.
This is how Dawn, who participated in the RESET-RA study, describes her personal experience with SetPoint Therapy: “I used to not be able to walk around the block, now I can walk for over an hour without any pain.”
Your doctor will review the risks associated with the SetPoint Therapy before referring you for the therapy. A SetPoint Activator can help you connect with a rheumatologist in your area who is offering SetPoint Therapy.
Click here to connect with a SetPoint Activator.
Actual patient stories shared with their permission. Individual outcomes vary. Click here to see the full Important Safety Information.
*Analysis based on patients who were treated with SetPoint Therapy for 12 months without the addition of biologic, JAK inhibitors or high-dose steroids, or change in csDMARDs.
How long does it take for the SetPoint System to work?
The level of effectiveness and time to effectiveness varies from patient to patient. In the RESET-RA study, improvement in the number of tender and swollen joints and disease activity was seen by 3 months after starting stimulation. This response rate was higher in patients who had exposure to just one prior biologic.
While results vary, this is how RESET-RA participant, Patty, describes her experience: “After the device was activated, within a month, I started noticing differences. I wasn’t as tired […] I think the biggest thing was I started doing things again.”
Hear more of Patty’s story.
Actual patient stories shared with their permission. Individual outcomes vary. Click here to see the full Important Safety Information.
What are the possible side effects of the SetPoint System?
The SetPoint System is placed on your vagus nerve during an outpatient procedure under general anesthesia. There are some risks associated with the device placement. Serious safety events related to the device, or its procedure, were observed in 1.7% (of the 242 study participants) in the RESET-RA study. All events were anticipated and consistent with learnings from more than 100,000 patients who have been treated with other vagus nerve stimulation devices for other indications over the past 20 years.
Other non-serious events, which typically resolve over time, include pain, redness and swelling at the implantation site, sore throat, cough or tingling as well as risks of vocal cord complications, that typically present as hoarseness – this was the most commonly observed side effect of the implant procedure occurring in about 5% (11 of 242 study participants).
Here is how Lynn, a RESET-RA study participant who experienced one of the more severe cases of hoarseness, described her experience with the side effect:
“It is normal to be hoarse right after surgery, but I couldn’t really speak. A whisper is all I could get out. So, I had to see a vocal cord specialist… I had a procedure done and the next day, I could talk like I had my voice back. Now, I can speak in a normal voice, and can be heard.
But, as I’ve told everyone, if I had lost my voice completely, I would still have the implant because not being able to talk is fine when all my pain was taken away. Knowing what I know now, and having the effects with the vocal cord, 100% would still do SetPoint, 100%.”
The SetPoint System activates your natural immune pathways to reduce inflammation and symptoms of disease without compromising your immune system – allowing for RA symptom reduction while preserving the body’s ability to fight infections. 5,6,7 In the 12 months of follow-up in the RESET-RA study, there were no serious infections, cancers, or major cardiac issues associated with the SetPoint Therapy.
Your doctors will discuss the risks of SetPoint Therapy with you.
Actual patient stories shared with their permission. Individual outcomes vary. Click here to see the Important Safety Information.
What should I expect before, during, and after the Implant placement procedure?
The first step of activating your SetPoint Therapy is to discuss this new therapeutic option with your rheumatologist or their team. To check if your rheumatologist offers SetPoint Therapy, please connect with the SetPoint Activator, or talk to your rheumatology provider.
Once you and your rheumatology care provider decide that SetPoint Therapy is the right option for managing your RA, they will refer you to a surgeon trained to place the SetPoint Implant. Next step would be to meet with the surgeon for a pre-operative visit to ensure that the procedure is right for you.
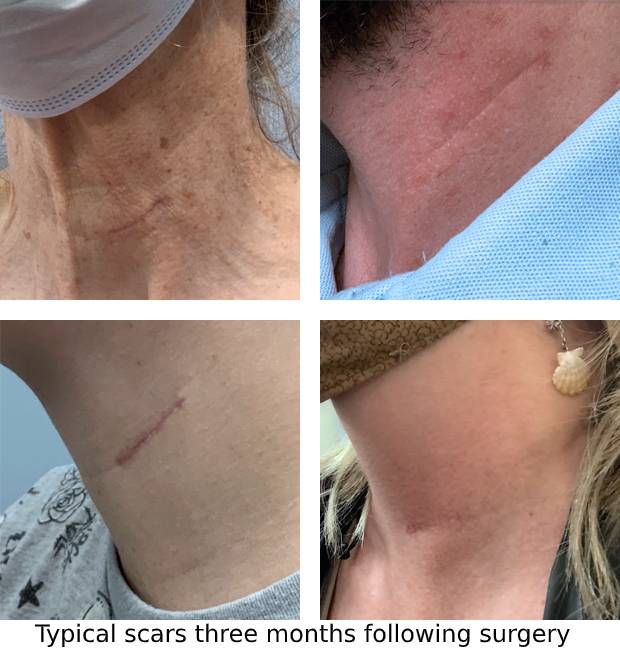
The actual placement procedure will be scheduled after your first surgeon appointment. The implant is typically placed during a 60-90 minute outpatient procedure, meaning that you typically return home the same day as the procedure and can resume activities of daily living the following day.8 The implant is placed via a small, 1-inch incision near a crease in the skin. You return to the surgeon a few days after the procedure for post-operative check. Click here to see examples of what the scar may look in patients who have had the SetPoint System.
A couple of weeks after the Implant is placed, you return to your rheumatologist who activates your therapy while ensuring that the stimulation is comfortable for you. Once activated, the therapy is automatically delivered daily. All you need to do is charge your device for a few minutes once a week. Your rheumatologist will continue to manage your RA. During these appointments, your therapy can be adjusted, if desired.
While results vary, this is how Lynn, a RESET-RA study participant, described the experience of her Implant placement procedure: “You’re implanted, your little scar heals, and you’re done. It was easily implanted. I had no downtime. I was literally in and out in the same day.”
Connect to a SetPoint Activator.
Actual patient stories shared with their permission. Individual outcomes vary. Click here to see the Important Safety Information.
Providers
How does the SetPoint System work?
The SetPoint System is a neuroimmune modulatory device that treats RA and is designed to restore immunologic homeostasis.2,4,8 The SetPoint System consists of an integrated neurostimulator intended to activate innate anti-inflammatory processes to modulate multiple intracellular pathways,2,4,5 thereby reducing production of an array of proinflammatory cytokines5,6—providing a novel treatment option indicated for your biologic-experienced RA patients. The stimulation parameters of the SetPoint System are based on extensive preclinical biomarker analysis that indicated that a single 60-second daily stimulation reduces multiple cytokines, including TNF, IL-1β and IL-6, by 30-70%, providing symptom reduction without immunosuppressive risks.2,4,5,6
Is the SetPoint System safe and effective for treatment of RA?
The SetPoint System’s unique mechanism of action builds on over two decades of research and engineering, including the clinical experience from other devices that stimulate the vagus nerve, to provide you and your patients with the first neuroimmune modulation therapy for treatment of RA.
The SetPoint System’s safety and effectiveness were studied in the RESET-RA clinical study that enrolled 242 patients who were inadequate responders or intolerant to one or more biologics and/or JAK inhibitors. The study successfully met its primary endpoint of ACR20 at 3 months, with sustained improvements observed through 12 months of follow-up compared to baseline. With 12 months of therapy, nearly half of the patients were in DAS28-CRP low disease activity or remission.*
The SetPoint System, as well as the implant procedure, is considered safe.9 There was a low risk (1.7%, n = 4) of related serious adverse event rate in the peri-operative 3 months, with no (0%) related serious events occurring through 12 months. Additionally, through 12 months, there were no malignancies, major cardiac events, or serious infections associated with the SetPoint System, highlighting the safety of this novel therapeutic option.
To see detailed data, visit the Clinical Evidence page.
*Analysis includes only patients who were treated for 12 months without addition of biologic/JAK inhibitors/steroids/change in csDMARD.
Who is the ideal patient candidate for SetPoint Therapy?
The SetPoint System is indicated for adults with moderately to severely active RA who have had an inadequate response, loss of response, or intolerance to one or more biological or targeted synthetic DMARDs.
Hence, the SetPoint Therapy could be considered for adult RA patients who have failed at least one biologic or JAK inhibitor and are seeking an alternative therapy; those who cannot tolerate these medications; those who are at high safety risks for JAK inhibition due to comorbid conditions; and those who had failed to get adequate disease control on biologics or JAK inhibitors.
In the RESET-RA study, improvement in the number of tender and swollen joints as well as disease activity was seen by 3 months after starting stimulation. This response rate was higher in patients who had exposure to just one prior biologic.
Based on its mechanism of action that manages the inflammatory process without immune suppression, SetPoint Therapy can be combined with biologics or JAK inhibitors if desired, preserving treatment options in the future.2,3,4,7
Do patients feel the implant or stimulation?
SetPoint Therapy is programmed to ensure comfort for the patients. Activation of the device includes a gradual increase of the stimulation level to ensure the therapy is not uncomfortable. Sensation of stimulation varies, but patients may feel a light tickling sensation, cough, or changes in voice during stimulation. The stimulation is delivered automatically for 60 seconds each day, and the rheumatologists can personalize the time of stimulation.
The SetPoint Implant typically cannot be felt or palpated externally.
What infrastructure does my clinic need to offer this to my RA patients?
We are committed to making it easy for you to offer SetPoint Therapy to your RA patients. Our Territory Manager will support your clinic’s onboarding process and can connect you with SetPoint-trained surgeons in your area.
Upon your clinic’s activation, SetPoint Medical will provide you and your team with the training and materials you’ll need to educate your RA patients on this therapy. SetPoint Medical will work directly with the surgical facility to help seek prior authorization for the procedure on your patient’s behalf. SetPoint Activators are available to help your patients navigate through the referral, prior authorization, and implant placement processes.
A couple of weeks after the implant procedure, the patient will return to your office to have therapy activated using an iPad-based app, which SetPoint Medical will provide. After activation, the therapy is delivered automatically for 60 seconds each day.
If you have additional questions, please contact your SetPoint Territory Manager
What is the implant procedure like?
The SetPoint System is typically placed during a 60-to-90 minute outpatient surgical procedure under general anesthesia.7 Patients typically go home the same day as the procedure and resume activities of daily living the following day.
The SetPoint Implant is placed by a surgeon who is experienced with procedures involving the vagus nerve, typically a functional neurosurgeon or an ENT surgeon. Your Territory Manager can help to identify trained surgeons near you. Additionally, SetPoint Medical will assist with the prior authorization process for the SetPoint procedure and will keep you and your patients informed to ensure a best-in-class experience for your patients and your staff.
If you have additional questions, please contact your Territory Manager
How does the vagus nerve control inflammation?
The vagus nerve plays a central role in modulating inflammation.8,9 Excessive inflammation plays a role in causing and advancing many autoimmune diseases, such as rheumatoid arthritis (RA). The sensory neurons within the vagus nerve detect and respond to inflammatory mediators by sending signals to the brainstem.8 Signals reflexively travel back into the body through motor fibers within the vagus nerve to enact the homeostatic control of both immune responses and restorative pathways through these linked innate neuroimmune reflexes.4,8
This pathway is thought to be dysfunctional in patients with RA causing increased inflammation that contributes to pain and swelling of the joints, putting patients at risk of joint deformities and disability if not well managed.3 Electrically stimulating the vagus nerve with precise parameters activates fibers of the vagus nerve that activate cholinergic anti-inflammatory processes that are intended to restore immunologic balance and reduce the release of inflammatory cytokines.2,4,10
How do you place the SetPoint Implant?
The SetPoint System includes a small, leadless, integrated neurostimulator that is placed directly on the left vagus nerve through a single incision during an outpatient procedure under general anesthesia. The implant is placed on the nerve within a flexible silicone Pod.
To implant the device, the vagus nerve is accessed, and then the Pod is placed under and around the nerve. The Implant is then placed in the Pod, which is then sutured closed holding the neurostimulator against the vagus nerve.
An integrated, leadless implant with rechargeable battery means there is no need to coil electrodes around the nerve, tunnel the lead, or to create a chest pocket for a separate battery.
Please see complete placement instructions in the Surgical Manual.
What patients are indicated for the SetPoint System?
The SetPoint System is indicated for adult patients with moderately to severely active RA who have had an inadequate response, loss of response, or intolerance to one or more biologic (e.g. TNF inhibitors) or targeted synthetic RA drugs (e.g. JAK inhibitors).
Can you tell me more about reimbursement for the SetPoint System?
Billing codes for the single incision implant, replacement, and removal procedures for the SetPoint System have been established. We have worked with the American Association of Neurological Surgeons (AANS), Congress of Neurological Surgeons (CNS), and American College of Rheumatology (ACR) to establish these CPT codes.
SetPoint Therapy is covered by insurances on a case-by-case basis, subject to prior authorization.
SetPoint Medical’s commitment to making SetPoint therapy accessible to patients means that we will work directly with the referring rheumatologist and the surgical facility to help seek prior authorization for the SetPoint Implant procedure on your patient’s behalf. You, your team, and your patients will be kept informed throughout the process to ensure a best-in-class experience.
How long does it take to see improvements in RA symptoms?
The SetPoint System is intended to improve RA by activating the innate anti-inflammatory and immune restorative pathways that reduce multiple cytokines, including TNF, IL-1β, and IL-6, by 30-70% to reduce inflammation without immunosuppression side effects.1,4,5,6
In the RESET-RA study, improvement in the number of tender and swollen joints and disease activity was seen by 3 months after starting stimulation. This response rate was higher in patients who had exposure to just one prior biologic.
Uniquely, due to its mechanism and safety profile, SetPoint Therapy can be combined with biologics or JAK inhibitors if desired, providing more treatment approaches to adequately control your patient’s disease.
To see detailed data, visit the Clinical Evidence page.
*Analysis includes only patients who were treated for 12 months without the addition of biologic/JAK inhibitors/high-dose steroids/change in csDMARD.
Connect With Us

2. Kelly MJ, Breathnach C, Tracey KJ, Donnelly SC. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep Med. 2022;3(7):100696.
3. Koopman FA, Tang MW, Vermeij J, et al. Autonomic Dysfunction Precedes Development of Rheumatoid Arthritis: A Prospective Cohort Study. EBioMedicine. 2016;6:231-237
4. van Maanen MA, Vervoordeldonk MJ, Tak PP. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat Rev Rheumatol. 2009 Apr;5(4):229-32.
5 Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity. 2017;46(6):927-942.
6. Koopman FA, Chavan, SS, Miljko A, Grazio Simeon, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis.
7. Peterson D, Van Poppel M, Boling W, Santos P, Schwalb J, Eisenberg H, Mehta A, Spader H, Botros J, Vrionis F, Ko A, Adelson D, Lega B, Konrad P, Calle G, Vale FL, Bucholz R, Richardson RM. Clinical safety and feasibility of a novel implantable neuroimmune modulation device for the treatment of rheumatoid arthritis: initial results from the randomized, double-blind, sham-controlled RESET-RA study. Bioelectronic medicine. 2024 10:8
8. Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853-859.
9. Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209(6):1057-1068.
10 Levine YA, Faltys M, Chernoff D. Harnessing the Inflammatory Reflex for the Treatment of Inflammation-Mediated Diseases. Cold Spring Harb Perspect Med. 2020;10(1):a034330.